Turn that Frown Upside Down
“I am honestly so impressed with the way my forehead lines have improved with Daxxify. I’d recommend to anyone!”
What is Daxxify®
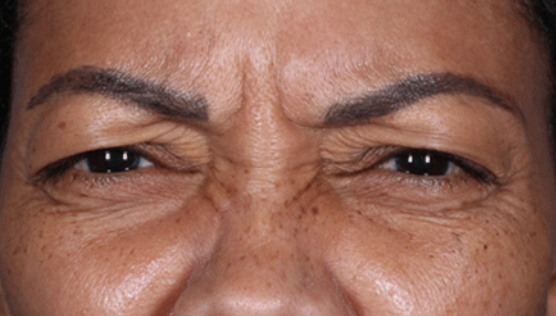
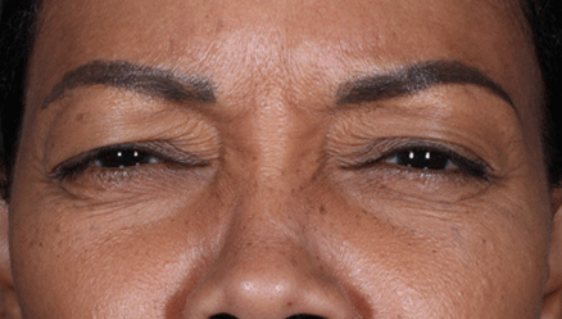
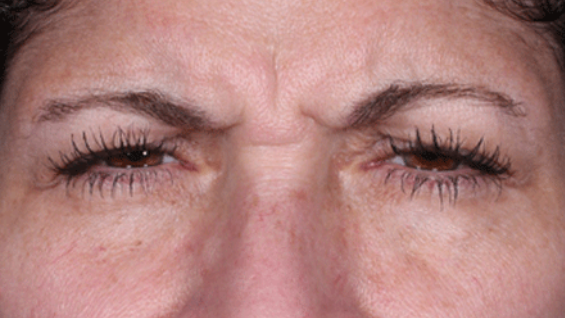
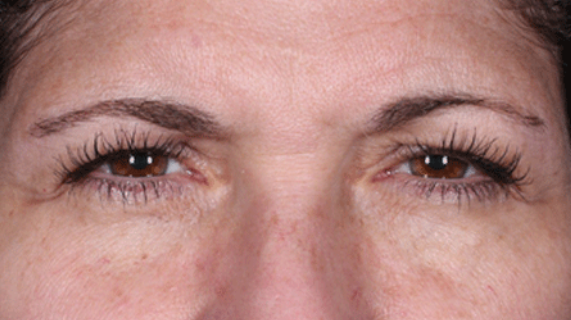
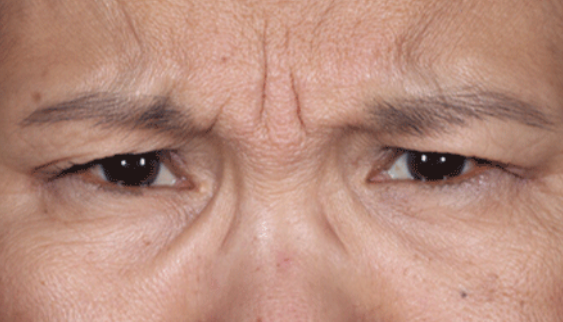
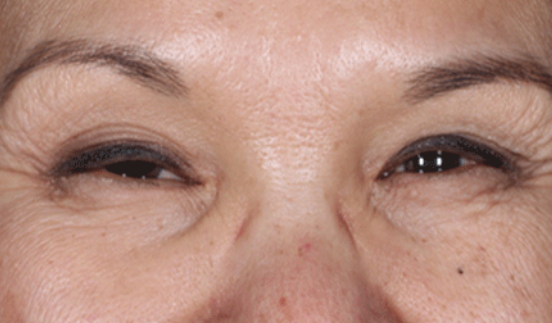
Daxxify is a peptide-enhanced injectable that treats glabellar lines, a.k.a. frown lines. It is the only prescription frown line treatment made in the United States. Created in innovative Silicon Valley, it continues to be manufactured there today. Daxxify contains a peptide exchange technology to deliver the botulinum toxin to the nerve cells so that it temporarily improves moderate to severe lines.
How is Daxxify® different from other treatments?
DAXXIFY® is the only long-lasting frown line treatment powered by a peptide. Results last on average 6 months and up to 9 months for some.
All frown line treatments require a special ingredient to stabilize botulinum toxin A, the protein responsible for helping smooth moderate to severe frown lines. For instance, BOTOX® Cosmetic uses human serum albumin (HSA), a blood product, as its stabilizer. Dysport®, another frown line treatment, uses HSA as a stabilizer and cow’s milk protein as a protectant.
DAXXIFY® is unique because it is the only formulation that uses a novel peptide as a stabilizer and does not contain human or animal byproducts.
FAQs
-
DAXXIFY® is FDA-approved to smooth moderate to severe lines between the brows. It is the only long-lasting frown line treatment powered by a peptide with results that last on average 6 months and up to 9 months for some.*
The active ingredient in DAXXIFY® is a purified protein called botulinum toxin type A.
-
Is your frown line treatment not lasting as long as you would like? If so, DAXXIFY® could be the solution for you.
-
All frown line treatments require a special ingredient to stabilize botulinum toxin A, the protein responsible for helping smooth moderate to severe frown lines. For instance, BOTOX® Cosmetic uses human serum albumin (HSA), a blood product, as its stabilizer. Dysport®, another frown line treatment, uses HSA as a stabilizer and cow’s milk protein as a protectant.
DAXXIFY® is unique because it is the only formulation that uses a novel peptide as a stabilizer and does not contain human or animal byproducts.
-
Your specialist will create a tailored treatment plan based on your chin profile. At each treatment, you will be given multiple small injections under your chin. You may receive up to 6 treatment sessions, spaced at least 1 month apart. Many patients experience visible results in 2 to 4 KYBELLA® treatments. 59% of patients received 6 treatments in clinical studies.
In clinical studies, results for KYBELLA®-treated patients who had
A ≥1-grade composite combined physician- and patient-rated improvement were 8% (1 treatment), 28% (2 treatments), 43% (3 treatments), 55% (4 treatments), 66% (5 treatments) and 72% (6 treatments), compared with 2% (1 treatment), 7% (2 treatments), 12% (3 treatments), 14% (4 treatments), 15% (5 treatments), and 22% (6 treatments) of patients treated with placebo
A ≥2-grade composite combined physician- and patient-rated improvement were 0% (1 treatment), 0.2% (2 treatments), 0.8% (3 treatments), 3.5% (4 treatments), 3.9% (5 treatments) and 15% (6 treatments) compared with 0% (1 treatment), 0.2% (2 treatments), 0.2% (3 treatments), 0.5% (4 treatments), 0.2% (5 treatments), and 1.5% (6 treatments) of patients treated with placebo
†Results from a survey of men to determine most likely treatment areas and top areas of concern for injectable-naive, aesthetically-oriented men aged 30 to 65 years (N=600). Proportion of respondents selecting that area as highest priority to treat using the Maximum Difference (MaxDiff) scaling system.
-
In short, no, and here’s why. Units are like fingerprints—they are a proprietary measurement of a treatment’s unique potency.
Because of the differences in how treatments are formulated and tested, you cannot compare
The most common side effects are swelling, bruising, pain, numbness, redness and areas of hardness around the treatment area. These are not all of the possible side effects of KYBELLA®. Call your healthcare provider if you develop open sores or drainage from the treatment area or for medical advice about side effects.
-
The safety and efficacy of DAXXIFY® is well established, with more than 20 years of research. In fact, DAXXIFY® has been studied in the largest-ever phase 3 clinical trial conducted for a frown line treatment and included more than 2,800 people across different ages and with different skin types. DAXXIFY® was studied in patients with varying skin types.